The Research Center for Natural Products & Drug Development (RCNPDD) provides professional services for herbal medicine development in order to shorten the development time, including: (1) Separation of key active ingredients, (2) Molecular screening and identification of Chinese herbal medicines, (3) Preclinical effectiveness assessment and best effective dose assessment, (4) Animal toxicity testing, and (5) Clinical trial assessment.
1. Separation of key active ingredients
The first task for the development of Chinese herbal medicines is to decode the composition of complex and diversified Chinese herbal medicines. There are many chemical properties of components that are very similar to those of Chinese herbal medicines, and effective key ingredients can be screened efficiently. RCNPDD provides advanced separation and purification technologies and equipment, and can further discuss the need to screen out the effective substances that need to be separated according to user needs.
2. Molecular screening and identification of Chinese herbal medicines
After separation and purification of the Chinese herbal extract, it is necessary to further identify thesubstancesand elucidate their possible structure. The Center has inherited many years of research experience from the Graduate Institute of Natural Products. Over the past decade, thousands of small molecules from Chinese herbs have been identified. The center provides a variety of molecular instrument identification services, combined with another technical service provided by the center to analyze the chemical fingerprints of Chinese herbal medicines, and uses professional, accurate and scientific identification methods to import all identified Chinese herbal medicine screening molecules into Taiwan Database of Extracts and Compounds (TDEC). This database can be used in the future to discuss and establish a cooperation with collaborators in order to develop a new drug.
3. Preclinical effectiveness assessment and best effective dose assessment
Our center provides pre-clinical cell experimental tests, microbiological tests, and animal experiments to test the best medicinal dose of the separated Chinese herbal medicines. The Center also provides a variety of Chinese herbal medicine functional reports that allow collaborators to select scientific reports for the required services.
4. Animal toxicity testing
The raw materials used for the separation and extraction of Chinese herbal medicines are often difficult to utilize due to their high toxicity, which makes them unable to be approved by the competent medical authorities as approved safe drugs. Toxicity testing is often an important indicator of clinical trials during the development of herbal medicines. The Center and related high medical research units have rich experience and cooperated for many years and can provide complete animal toxicity testing to provide a comprehensive toxicological assessment report for partners.
5. Clinical trial assessment
The Center's R&D team cooperates with resident physicians in the Kaohsiung Medical University Hospital of various departments for many years and will be able to provide partners with more in-depth clinical trial cooperation opportunities in the future. However, clinical trials need to be agreed with doctors through the Human Trials and Research Ethics Committee to conduct clinical trial assessment tests. Therefore, RCNPDD will assist users by conducting preclinical laboratory assessments or reviewing all preclinical statutory laboratory reports, and then will help to inform the doctors collaborating with the Center to conduct relevant clinical trials.
Chinese herbal medicines are usually complex and are composed of a number of compounds. In order to establish internationally certified standard analysis methods and identification procedures, RCNPDD introduced the concept of “fingerprints” to reintegrate the specificity of Chinese herbal medicines and to establish a scientific analysis process for Chinese herbal medicine extracts. The analytical methods of the chemical fingerprints provided by the Center are (1) Chromatographic analysis, (2) Spectroscopic analysis, and (3) Other analytical methods.
1. Chromatography analysis (TLC, HPLC, CE, etc.)
RCNPDD provides thin layer chromatography (TLC), high-performance liquid chromatography (HPLC), and capillary electrophoresis (CE) techniques. For the crude extract of Chinese herbal medicine, the initial separation of components from the crude extracts is established by thin layer chromatography, and the values of the distances between the specific components and the solvent are calculated to determine the preliminary chemical analysis conditions for chemical crude extracts of Chinese herbs. The Center also provides high-performance liquid chromatography instrumentation to separate and identify the crude extracts of Chinese herbal medicines and use the separation conditions under different solvent conditions as the basis for the chemical fingerprints of Chinese herbal medicines and the basis of Chinese medicine quality control.
2. Spectroscopic analysis (UV, IR, Near-IR, etc.)
The center also provides instruments for spectroscopic analysis such as UV, IR, Near-IR and other specialized analytical instruments to establish the specific absorption wavelengths of Chinese herbal extracts.
3. Other analytical methods (X-ray, NMR, GC-MS, LC-MS, etc.)
The center also uses X-ray, NMR, GC-MS, LC-MS to analyze the chemical structure, properties and molecular weight of small molecules from Chinese herbal extracts. Extracts of Chinese herbs are also analyzed by NMR spectra, using PCA (multivariate analysis), construction of chemical map data, and analysis using Bruker's machines. Other detailed services are welcome to be requested and to discuss further cooperation within our Center (see "Contact Us").
The Case Study of Improving the Prognosis of Second and Third Stage for Breast Cancer Patients by Chinese Herbal Capsules
The cooperation of the following study was initiated by the research team of RCNPDD and its Director Wu Yang-Chang, together with Professor Hou Ming-Feng's team of breast surgery at the attached hospital of Kaohsiung Medical University and Professor Chen Zhong-Ren of the Department of Traditional Chinese Medicine, and aims to test the drug commissioned by the GMP Chinese medicine factory to manufacture the capsule.
This study investigated the effect of Chinese medicine capsules on improving the prognosis of second and third stage breast cancer patients. Breast cancer can be divided into stage 1 to stage 4 breast cancer depending on the size of the tumor, carcinoma in situ, local lymph node invasion, and distal metastasis. The treatment methods also differ according to different stages. The treatment of orthotopic breast cancer is performed by surgery. Treatment is mainly supplemented by radiation therapy, and no post-operative chemotherapy is required, but regular follow-up is required. The first and second stage breast cancer patients without lymph node metastasis can be treated according to the type of breast cancer after the surgical treatment and regularly tracked; if it is a patient with lymph node metastasis, postoperative chemotherapy must be supplemented and anti-hormonal therapy drugs should be given according to the type of breast cancer. At the third stage, the breast cancer is too large and it is not easy to remove, which leads to the proliferation of cancer cells. Therefore, chemotherapy will be given before surgery to reduce the tumor size and then to eliminate it. The fourth stage of breast cancer is when breast cancer cells pass through the lymphatic system and are transferred via blood system to the bones, lungs, ribs and other internal organs. The chance of healing is small but can be relieved by hormones and chemical anti-cancer drugs supplemented by surgery or radiation therapy to prolong life.
According to the results of the 2000-2011 survey conducted by the Health Insurance Database, it was found that the group of people newly diagnosed with breast cancer who had taken Chinese medicine for more than 30 days had a good chance of reducing the risk of death from breast cancer, and the risk was further lowered with the increase in the number of days and dose; another Chinese medicine composition made according to a specific ratio had the ability to significantly reduce cell proliferation and metastasis, and significantly reduced the chance of distant metastasis in animal experiments.
Therefore, this study intends to study the changes or benefits of breast cancer patients who were diagnosed with breast cancer in the second and third phases of the operation after taking additional Chinese herbal capsules for six months (3 each morning, afternoon, and dinner). Therefore, the blood analysis basic value of the subject will be taken before the test. After taking the Chinese medicine capsule for six months, the subject will be selected again for blood analysis and the changes between the two will be compared. Then there is a time for five-year follow-up period. During the follow-up period, the blood will be drawn every six months. Blood tests for CA153 and CEA indices to monitor the progression of the tumor course.
RCNPDD provides a variety of bioinformatics analysis methods such as molecular docking, molecular modeling, virtual screening, and quantitative structure-activity relationship (QSAR).
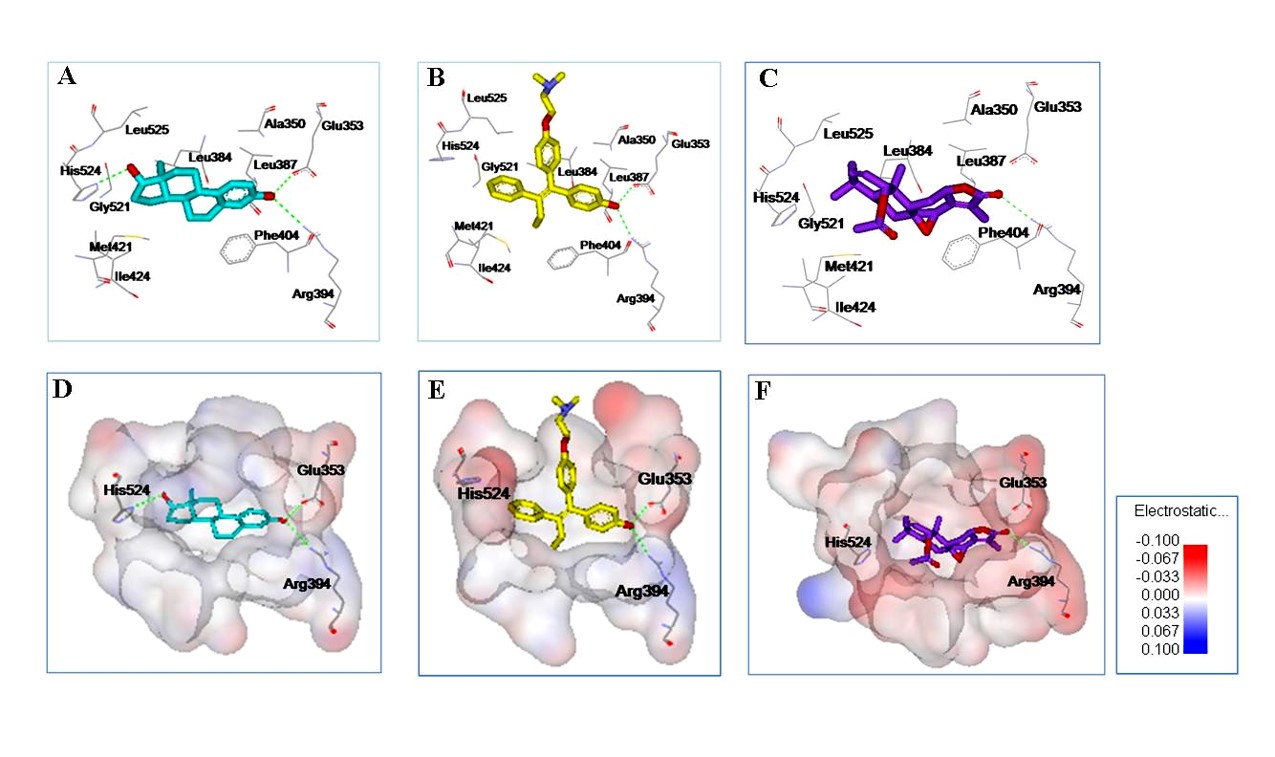
1. Molecular Docking
RCNPDD can use a variety of small molecule docking calculation software such as AutoDock, iGEMDOCK, GOLD and other software to perform molecular docking calculation. The interaction between the molecule and target protein will be understood by molecular modeling analysis.
Molecular docking can provide molecular biological information such as number of hydrogen bond acceptors or donors, the polar or non-polar interactions, binding affinity and so on. This information can also provide structurally-based optimization strategies for small molecule drug design.
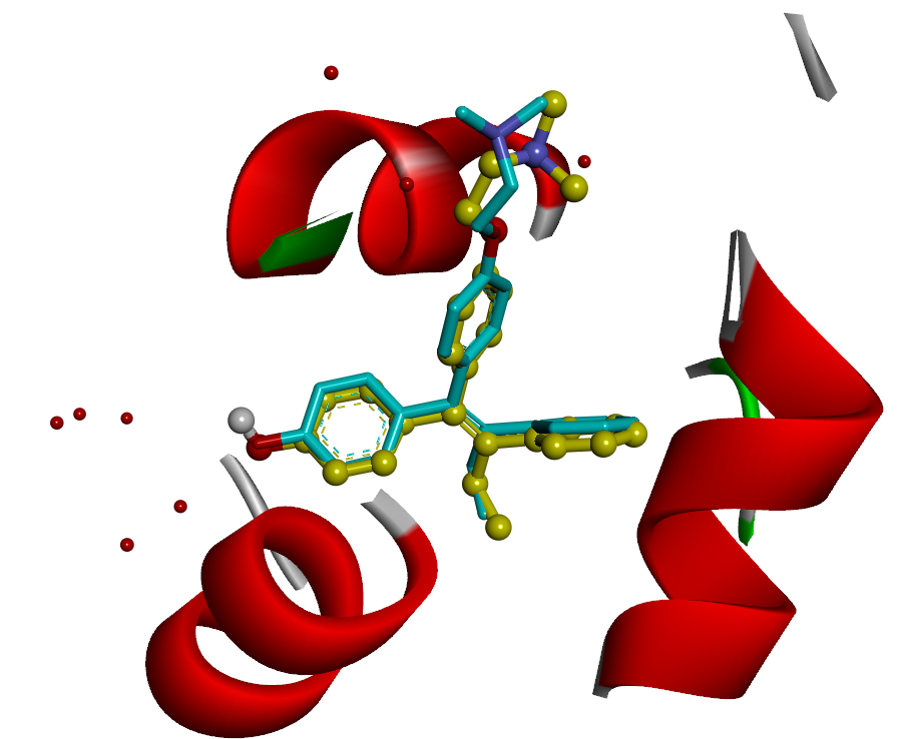
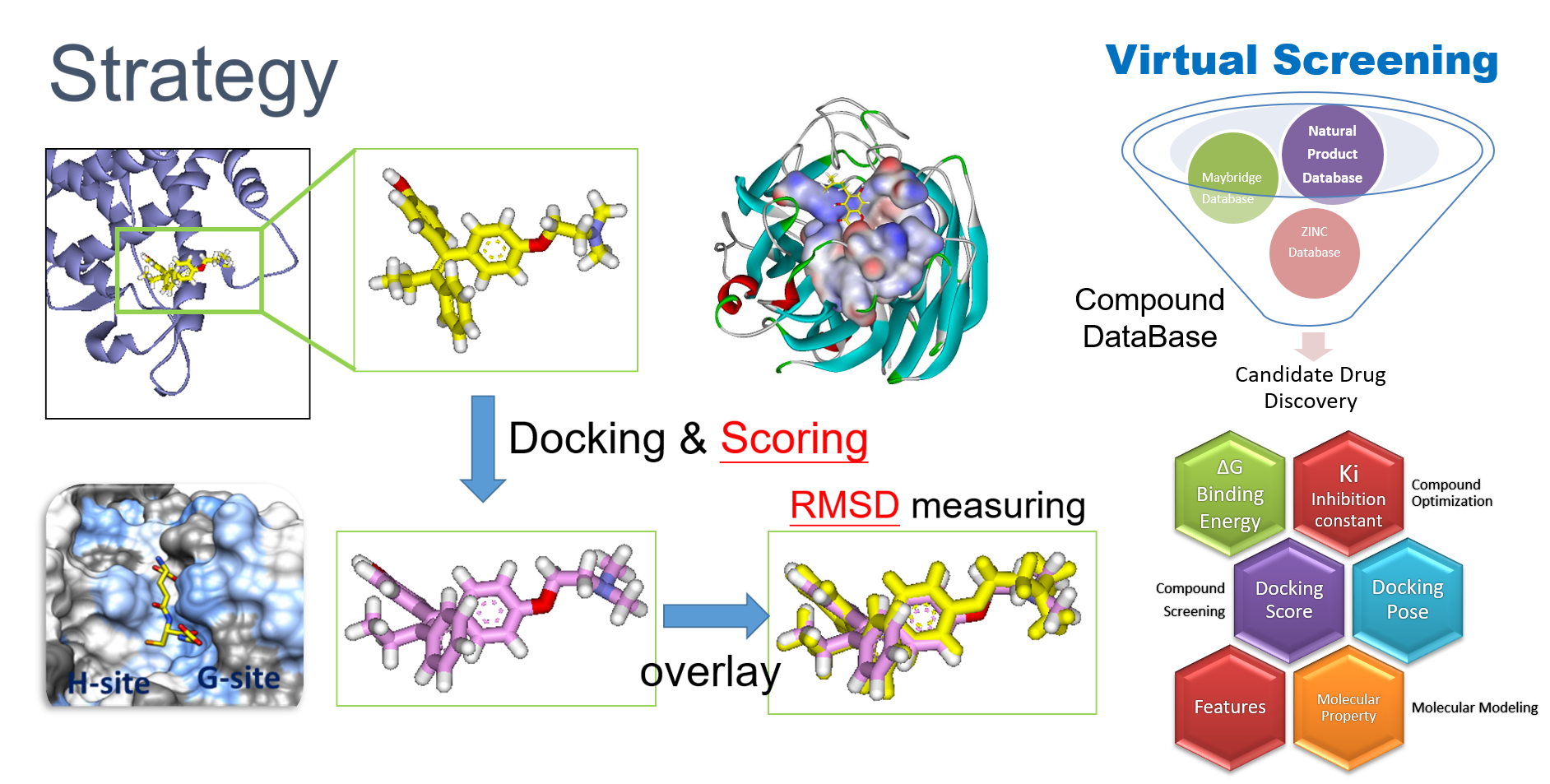
2. Virtual Screening
RCNPDD can use a variety of docking software to perform virtual screening technique to dedicate to find potential drug from small molecular databases (eg. ZINC Database).
Virtual screening technology had high speed and low-costadvantages for drug discovery from a large scale database. The technique can help biomedical researchers find potential candidates which focus on specific target protein structures. The small molecule can also carry out further subsequent biological activity experiments such as anti-cancer, anti-inflammation and etc. The researcher can also further perform analysis to do signal pathway discovery.
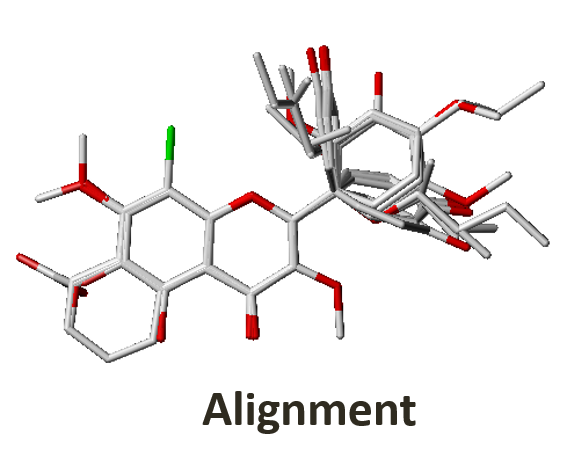
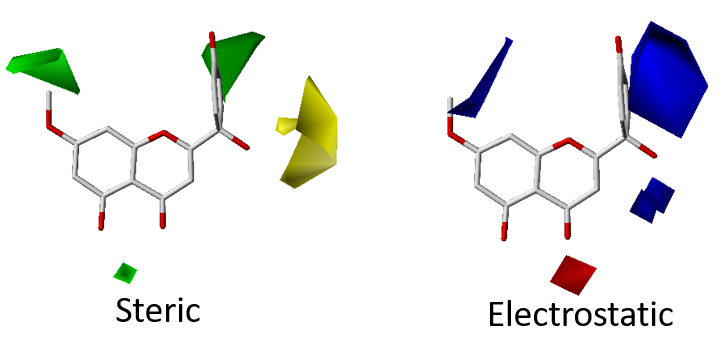
3. Quantitative Structure-Activity Relationship (QSAR)
RCNPDD can use the SYBYL software for 3D QSAR (The software service needs legal registration via National High Speed and Computing Center). 3D QSAR is a ligand-based drug design tool which focuses on understanding the relationship between structure and activity. The CoMFA and CoMSIA simulation models can provide some information to researchers. These models can offer structure or electronic based suggestions to the researcher. It can help us focus on structure optimization and drug development strategy to shorten discovery time.